Drug Trials Snapshot: Zontivity (vorapaxar)
Disclaimer: The Drug Trials Snapshot provides information about who was in the clinical trials that led to the FDA approval of this drug. This website shows who participated in these studies by sex, race, and age groups. You can get more information about this drug from the ZONTIVITY Drug Label and your doctor or health care professional.
ZONTIVITY (vorapaxar)
zon-TIV-it-ee
Merck & Co., Inc.
Approval date: May 8, 2014
DRUG TRIALS SUMMARY
What is the drug for?
ZONTIVITY is used to treat people who have had a heart attack or reduced blood flow in their legs (peripheral arterial disease, PAD). ZONTIVITY is used with aspirin and/or clopidogrel to lower the patient’s chance of having another serious problem with their heart or blood vessels, such as heart attack, stroke, or death. It must not be used in people with a prior stroke, transient ischemic attack (TIA) or intracranial hemorrhage, because ZONTIVITY increases the risk of bleeding in the brain in these patients.
How do I use this drug?
ZONTIVITY is a pill taken once a day along with aspirin and/or clopidogrel.
What are the benefits of this drug?
Studies showed that ZONTIVITY reduced the rate of heart attack, stroke and cardiovascular death when compared to a placebo pill. It also reduced the need for emergency procedures in order to improve blood flow to the heart.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: ZONTIVITY is similarly effective in men and women.
- Race: Subgroup analyses were conducted, but the number of patients in the non-Caucasian subgroups was limited. Therefore, differences in response to ZONTIVITY by race could not be determined.
- Age: ZONTIVITY is similarly effective across all age groups studied.
What are the possible side effects?
The most common side effect of Zontivity during clinical trials was bleeding, which was observed in 25% of subjects taking ZONTIVITY compared to 17.6% of subjects who were given a placebo pill (see Table 5). More severe bleeding - bleeding requiring a transfusion - was reported in 3% of patients taking ZONTIVITY and 2% of patients taking placebo. Less common side effects included anemia, depression, and rash.
ZONTIVITY increases the risk of bleeding. Therefore, do not take ZONTIVITY if you have had a stroke or “mini stroke” (also known as transient ischemic attack, or TIA), have had bleeding in your brain, or currently have unusual bleeding, such as bleeding in your head, stomach or intestines (an ulcer.)
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of bleeding is higher among women taking ZONTIVITY than men.
- Race: There were too few non-Caucasian patients to make a reliable assessment of bleeding risk by race.
- Age: ZONTIVITY increases the risk of bleeding in all age groups. Because older patients have a higher risk of bleeding in general, there is more bleeding in older patients taking ZONTIVITY.
WHO WAS IN THE STUDY?
Who participated in the clinical trial?
The FDA approved ZONTIVITY based on evidence from a clinical trial study of 26,449 patients with a history of heart or blood vessel conditions. The studies were conducted in North America, Europe, Latin America, Asia/Pacific/Australia/New Zealand, South Africa, and Israel.
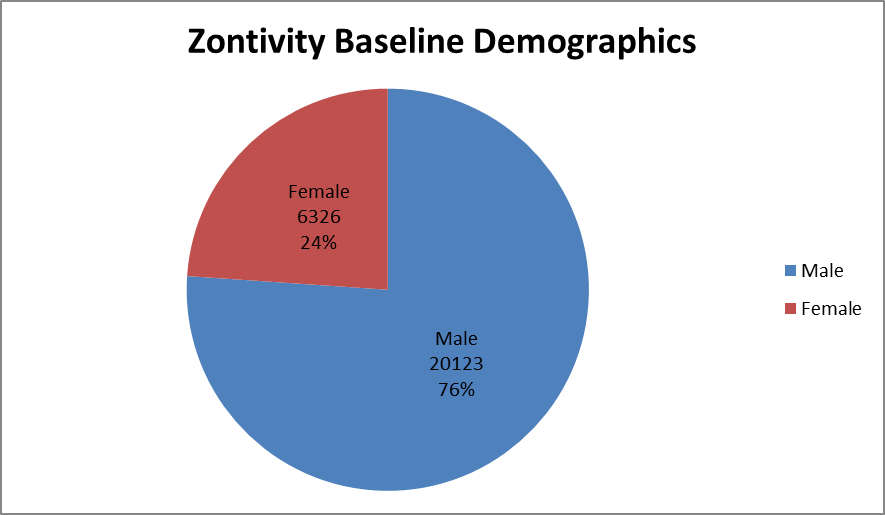
Figure 1 summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Medical Review, Table 24
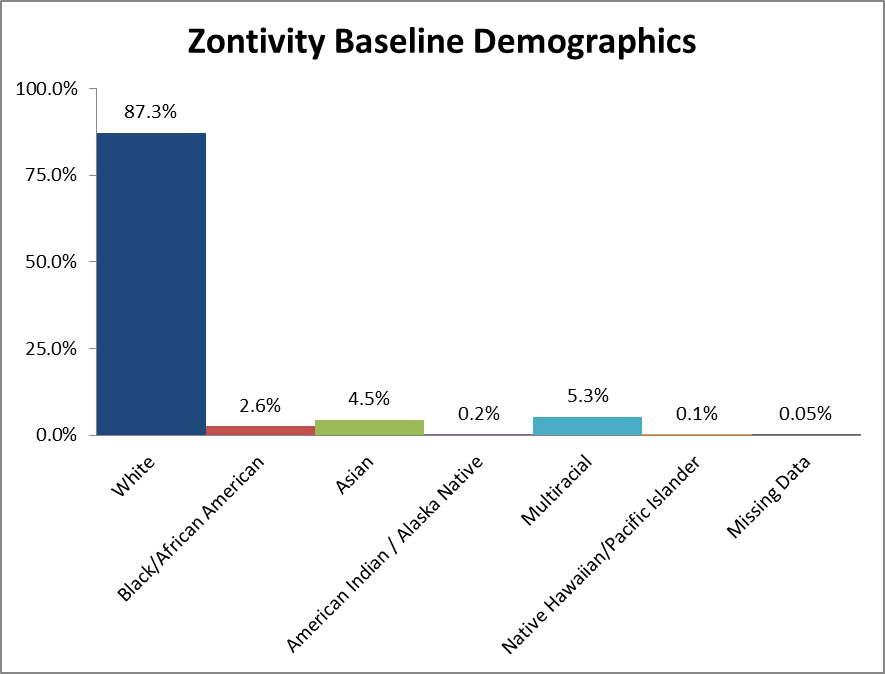
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Medical Review, Table 24
Table 1. Baseline Demographics by Race for the Intent to Treat (ITT) Population
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 23086 | 87.3% |
| Black/African American | 689 | 2.6% |
| Asian | 1194 | 4.5% |
| American Indian / Alaska Native | 49 | 0.2% |
| Multiracial | 1390 | 5.3% |
| Native Hawaiian/Pacific Islander | 29 | 0.1% |
| Missing Data | 12 | 0.05% |
Source: Adapted from FDA Medical Review, Table 24
How was the study designed?
ZONTIVITY was approved by the FDA based on a clinical study (TRA 2○ P - TIMI 50, Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events) of 26,449 patients with a history of myocardial infarction (MI), ischemic stroke, or peripheral arterial disease (PAD). Half the patients were randomly assigned to ZONTIVITY and half were assigned to placebo. Neither the patients nor the health care professionals knew which patients were taking ZONTIVITY and which ones were taking the placebo until after the drug trial was complete. Along with the ZONTIVITY or placebo, all patients were to take aspirin, clopidogrel, or both drugs, and about three-fourths of patients took both. Patients were followed for up to four years. The primary endpoint of the study was the time to first heart attack, stroke, cardiovascular death, or urgent procedure to improve blood flow to the heart (coronary revascularization). An interim analysis revealed that patients who had experienced an ischemic stroke or TIA prior to entry into the study had an unacceptably increased rate of intracranial hemorrhage, so a decision was made to discontinue these patients from the study. Analyses were carried out on all patients randomized, (26,449) but also on the subset of patients with MI and PAD who had not experienced a stroke or TIA prior to study entry (20,170).
What are the results of the efficacy study?
ZONTIVITY, taken with aspirin and/or clopidogrel, reduced the rate of heart attack, stroke, cardiovascular death, and urgent procedures to improve blood flow to the heart (coronary revascularization) when compared to placebo.
What are the results of the safety study?
Patients taking ZONTIVITY experienced increased moderate or severe bleeding events by 55%.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
MEDICAL REVIEW (PDF - 14.96MB)
DRUG LABEL (PDF - 698KB)
OFFICE DIRECTOR MEMO (PDF - 1.25MB)